Abstract
Introduction: In the ASPIRE and ENDEAVOR trials, multiple myeloma (MM) patients treated with carfilzomib (K) had significantly improved progression‐free survival and overall survival compared with standard of care. The incidence of all-grade adverse cardiovascular events (ACVE) was 26.6% and 24.5% in the K treated groups in ASPIRE and ENDEAVOR respectively. The atherosclerotic cardiovascular disease (ASCVD) score is a risk stratification tool used by cardiologists to classify patients with a score ≥7.5% as high risk for the incidence of ASCVD events. To date, there are no clinically relevant models that predict the likelihood of ACVE in MM patients treated with K nor are we aware of any protective factors against ACVE. Our study aims to identify factors which can predict and mitigate the incidence of ACVE.
Methods: A retrospective chart review of 372 MM patients who were treated with K between 2011 and 2018 at our institution was performed.Data were summarized using descriptive statistics; Chi-square tests of association and Kruskal-Wallis tests were employed to test for differences across groups, where appropriate. Logistic regression was performed to examine the risk factors associated with ACVE.
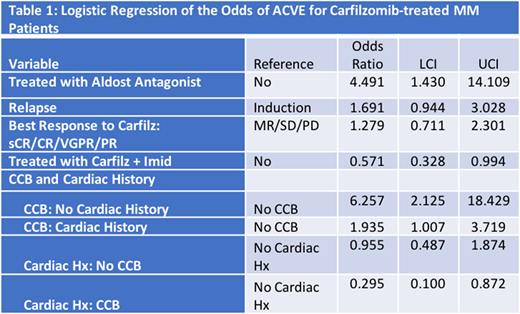
Results: Of the 372 patients, 243 (65%) were Durie-Salmon Stage (DSS) III, 70 (19%) were International Staging System (ISS) III; 186 (50%) were men. There were a total of 102 (27%) ACVE and 33 (9%) ≥grade 3 events. The most common ACVE were congestive heart failure (29%), asymptomatic elevations in pulmonary arterial systolic pressure (PASP) (27%), and hypertension (HTN) (16%). Sixty-eight (66%) Caucasians, 14 (14%) African Americans and 17 (17%) Hispanics developed ACVE. Aspirin (ASA) (64%), beta blockers (BB) (51%), calcium channel blockers (CCB) (43%) and statins (37%) were the most common cardiac medications patients were taking at the time of K initiation. Regardless of cardiac history, patients who were taking a CCB had higher odds of experiencing an ACVE compared to patients who were not taking a CCB (CCB + Cardiac history: OR=1.9; CI=1.0-3.7; CCB, no Cardiac history: OR=6.3; CI= 2.1-18.4) (Table 1).
Patients with an ASCVD score ≥7.5% did not have an increased incidence of ACVE but did have an increased incidence of ≥grade 3 vs. grade 1-2 ACVE (100% vs. 78%, p=0.092). Prior cardiac history, including history of HTN, was common (59%) but not associated with incidence or severity of ACVE. Those with type 2 diabetes had a higher rate of ≥grade 3 vs. grade 1-2 ACVE (33% vs. 10%, p=0.004).
Patients on certain CV medications while receiving K-based treatment had a higher incidence of ACVE compared to those not on these medications, including BB (51% vs. 34%, p=0.002), CCB (43% vs. 26%, p=0.002), aldosterone antagonists (AA) (8% vs. 3%, p=0.024) and statins (37% vs. 25%, p=0.022). Patients on ASA were more likely to experience ≥grade 3 compared to grade 1-2 ACVE (78% vs. 53%, p=0.016). ACE inhibitors or angiotensin receptor blockers (ACE/ARB) were not associated with increased incidence or severity of ACVE.
The use of K in relapsed, as opposed to induction setting, was associated with a higher incidence of ACVE (31% vs. 20%, p=0.035). Maximum dose and number of K doses were not associated with ACVE. There was no relationship between PASP elevation and age >75, use of BB, CCB, AA, ACE/ARB, number of K treatments or maximum dose of K.
Factors that were associated with increased odds of ACVE in multivariate analysis included patients who received K in the relapsed setting (OR= 1.7; CI=0.94-3.0) and use of an AA (OR= 4.5; CI=1.4-14.1) (Table 1).
Conclusion: In line with prior studies, 28% of MM patients treated with K at our institution developed an ACVE, with 9% grade 3 or above. Use of CCB and AA were particularly associated with increased risk of ACVE, while use of ACE/ARB were not. ASCVD score ≥7.5% was associated with an increased incidence of ≥grade 3 ACVE in K-treated patients and should perhaps be evaluated as a predictive tool in prospective studies.
Siegel:Celgene: Consultancy, Honoraria, Research Funding, Speakers Bureau; Janssen: Consultancy, Honoraria, Speakers Bureau; Takeda: Consultancy, Honoraria, Speakers Bureau; Novartis: Honoraria, Speakers Bureau; Amgen: Consultancy, Honoraria, Speakers Bureau; BMS: Consultancy, Honoraria, Speakers Bureau; Karyopharm: Consultancy, Honoraria; Merck: Consultancy, Honoraria, Speakers Bureau. Biran:Merck: Research Funding; Amgen: Consultancy, Speakers Bureau; Takeda: Consultancy, Speakers Bureau; Celgene: Consultancy, Honoraria, Speakers Bureau; BMS: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal